The radiative impacts of the two gases differ, with much stronger impact initially from the methane, per gigaton of carbon (Gton C) released. The difference is due to the different starting concentrations in the atmosphere. There is a lot more CO2 than methane in the air, so the absorbtion bands for CO2 are more saturated, and so the impact of another Gton C is less for CO2.
The gases also differ in their atmospheric lifetime, with methane breaking down (to CO2, which effect is included in the model) in about a decade. The uptake of CO2 by ocean dissolution and ultimately chemical weathering reactions is somewhat more complicated, but it has a long time scale.
The temperature evolution is controlled by two heat reservoirs: a shallow and a deep ocean. The shallow ocean equilibrates to the radiative forcing in about a decade, and it also exchanges heat with the deep ocean, which sets the pace for the temperature change of the Earth overall. The buildup of long-term "heat pollution" on the planet is simulated by the deep ocean temperature anomaly.
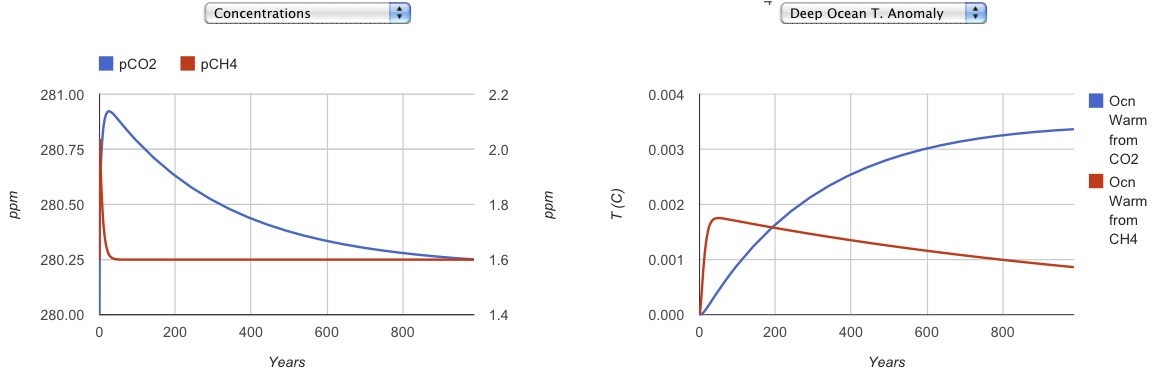
One gigaton of each gas is released at year zero. The concentrations, radiative impacts, and temperature impacts of the atmosphere and the deep sea are all tracked through time.
Compare the radiative forcings for the two gases by selecting that option in the menus just above either plot. Increase the CO2 spike if you like to equalize the radiative forcings of the gases immediately after their release in year 0. How many CO2's is one methane worth in immediate radiative forcing?
The equilibrium temperature anomaly is the temperature that the instantaneous climate forcing, times the climate sensitivity, aspire to. However, there are time constants for changing the temperature of the Surface (about 10 years), and the deep sea (about 1000 years). Hence these temperatures equilibrate slowly.
Be sure to track the progress of the climate perturbations from CO2 and methane by zooming out in time, using the Show menu at the page bottom.
The amount of energy gained by combustion of coal to make the CO2 slug, or the amount we would have gotten from combusting the methane rather than releasing it, are compared with the time-integrated radiative forcings from the releases of the greenhouse gases, in units of 1021 Joules. Talk about unintended consequences!
| Compare the lifetimes of the two greenhouse gases in the atmosphere. |
| Compare the instantaneous radiative forcings of the gases, by increasing CO2 until the initial RF values are the same. |
| Compare the temperature impacts of the two gases at times of 40 years (about when we might start to hit 2 degrees C, i.e. global warming happens), and 1000 years |
| Compare the useful energy yield from fossil fuel combustion to the energy trapped by the greenhouse gases over time. |
 |
The University of Chicago 5801 South Ellis Ave Chicago IL 60637 773.702.1234 |